Projects
Causal estimation of intervention effects based on observational data in a University Hospital setting with an example from septic shock patients treated with therapeutic plasma exchange as a case study
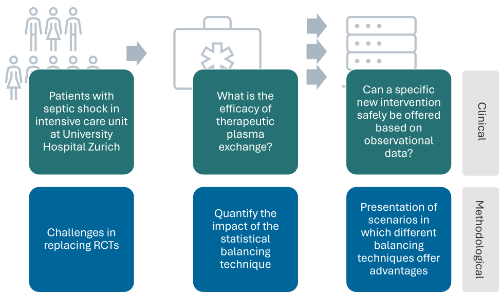
The simulation study will reveal which balancing methodology is robust to model misspecification in
the presence of correlated covariates and confounders. Through the variation of relevant factors in
the simulation study design, high-level evidence can be drawn, and the results will be generalisable
from intensive care medicine to other clinical applications with similar challenges. In the intensive
care setting, the results would elucidate evidence-based indications for the use of off-label therapies.
Project lead: Ulrike Held & Pedro David Wendel Garcia (USZ)
Funding: Stiftung für Wissenschaftliche Forschung an der Universität Zürich
Statistical considerations regarding transportability of clinical prediction models
In transplantation medicine, relevant outcomes include organ/graft survival and overall survival. For most organs, the number of patients in need of an organ exceeds the number of donors. In this context, the use of prognostic models is of high relevance from a patient perspective, because these mathematical models should guarantee that the best combination of donor and recipient can be found.
This master thesis aims to explore the consequences of deficiencies in the development phase of the prediction model. The first objective is to assess the impact on model transportability when a highly discriminative prognostic factor in one setting proves less relevant in another. For instance, in the UK DCD Score, "retransplantation" is a key prognostic variable, whereas, in Switzerland, retransplantation of donation after circulatory death (DCD) livers is rare. The second objective is to evaluate the implications of using a reduced model without refitting the coefficients. For example, despite substantial model reduction, the Kidney Donor Risk Index (KDRI) still uses the coefficients from the original model. The results of the master thesis will be highly relevant for clinical practice in the context of organ transplantation in Switzerland.
Project lead: Ulrike Held & Simon Schwab (Swisstransplant and CRS)
Master Student: Damian Brülhart
Funding: Internal
Causal evidence from observational data regarding the effect of initial opioid dose concentration on adverse outcomes - a study based on the target trial emulation framework
The target trial emulation framework was used to feature a randomized controlled trial aiming at addressing the research questions whether there is a causal relationship between the initial opioid dose and adverse outcomes in younger patients with musculoskeletal disorders. Real-world data was used based on claims from Helsana insurance company. Primary outcome was a composite outcome of death, emergency department visit, and hospitalization in an acute hospital. Further outcomes were burden of analgesic use, recurring opioid treatment, and duration of opioid treatment.
The protocol of the study can be found on OSF: here
The study was initiated as a master thesis of the Biostatistics master program.
Project lead: Ulrike Held & Maria Wertli (Kantonsspital Baden, Uni Bern)
Evidence synthesis methods in observational studies
In this methodological evaluation of different heterogeneity variance parameter estimates for random effects meta-analysis, we aimed to compare different approaches, and quantify the effect of the heterogeneity measures. Outcomes addressed include binary outcomes, as well as continuous outcomes. (Master thesis: Stefania Iaquinto)
Project lead: Ulrike Held
Hypothermic oxygenated perfusion (HOPE) against cancer recurrence in HCC liver transplantation
Background to the research: A frequent and increasing indication for liver transplantation is the presence of hepatocellular carcinoma (HCC) in a cirrhotic liver. The shortcoming, however, of including patients with HCC is tumor recurrence after liver transplantation. This occurs in approximately 15 % of cases, and is known to potentially correlate with donor liver quality. Machine liver perfusion, as for example hypothermic oxygenated liver perfusion (HOPE), is a novel method to optimize the quality of donor livers before implantation compared to the standard procedure, which is cold ice preservation.
The aim of our study is therefore to investigate, if patients with HCC have less HCC recurrence by donor liver treatment with HOPE.
The study appears timely, as HOPE is an easy and short term machine perfusion treatment of donor livers for two hours before implantation. The study is also novel, as anti-tumor effects of HOPE have not been explored in a randomized manner. If HOPE has a benefit on tumor recurrence, we expect relevant consequences for clinical practice.
Design and methods used: We plan to recruit 220 patients with liver cirrhosis and HCC listed for liver transplantation in 15 well known transplant centers. Patients will be randomized to HOPE treatment or standard cold ice preservation. We will compare time to eventual tumor recurrence or death (primary endpoint) between the two treatment groups (primary objective of this study). Secondary endpoints will include initial graft quality and liver graft injury during implantation, as well as complications after liver transplantation. The follow up period of this study will be 2 years, as most HCC recurrence after liver transplantation is kown to occur within this time frame.
Patient and public involvement: Liver transplant patients were involved in the study design and methods. They will also participate in site visits during recruitment and after completion of the study to share their experiences and the trial results with the centers and the public.
Project lead: Stefanie von Felten & Philipp Dutkowski (Clarunis Basel)
Funding: SNSF - Investigator initiated clinical trials (IICT) programme
Missing value imputation for clinical prediction models
Missing data are a common problem in the development of prediction models for clinical research. There are ways to handle missing data and some of them are based on variants of multiple imputation by chained equations, while others are based on single imputation. We aimed to investigate by simulation if some of these methods consistently outperform others in performance measures of clinical prediction models. We designed an extensive simulation study following the ADEMP framework to address this question.
The paper was published in the Journal of Clinical Epidemiology : https://www.sciencedirect.com/science/article/pii/S0895435624002956
The simulation study R code and graphical abstract of the manuscript was made available on OSF: here
Project lead: Ulrike Held
Phase III randomised controlled trial comparing maintenance with the addition of local ablative treatment for patients with advanced stage IV non-small cell lung cancer (salVage-Trial) – with SNSF funding for an IICT
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death worldwide. Up to 70% of all NSCLC patients present with already metastasized stage IV and are treated with systemic therapy alone as the current standard of care (SoC). Despite improvement of systemic therapy with excellent response rates, the median progression free survival (PFS) ranges between 5 to 25 months. To date, there are no recommendations about additional treatments for residual disease in patients responding to initial systemic therapy. Such approaches, named “local ablative therapy” (LAT), comprise surgical resection and/or radiotherapy to all residual lesions. Several retrospective cohort studies have documented promising outcome of so-called “salvage surgery” in these situations with median overall survival (OS) of 9 to 75 months, 5-year survival rates of 20-75% and increased PFS ranging between 5 and 43 months.
Rationale and aim: We hypothesize that LAT of NSCLC patients upon response to initial systemic treatment to an oligopersistent diease state will lead to an improvement of patients’ PFS with at least maintained QoL at six months from randomization.
Methodology: To confirm our hypothesis, a multicentre prospective RCT will be performed to assess two co-primary endpoints: PFS calculated from randomization and QoL measured with patient-reported outcome measures (PROMs) at six months from randomization using the EQ-5D-5L score with a hierarchical ordering.
Project lead, Methodology and Statistics: Ulrike Held & Priska Heinz
Funding: Swiss National Science Foundation
Preoperative smoking cessation program in patients undergoing intermediate to high-risk surgery
Background: At present, effectively implementing smoking cessation programs in the health care system constitutes a major challenge. A unique opportunity to initiate smoking cessation focuses on smokers scheduled for surgery. These patients are not only highly motivated to quit smoking but also likely to benefit from a reduction in postoperative complications which may translate into a decrease of costs. Nevertheless, surgical patients are not routinely informed about the benefits of preoperative smoking cessation. Potential reasons for this missed opportunity may be the lack of time and training of surgeons and anaesthesiologists. We therefore aim to analyse the impact of a preoperative high-intensity smoking cessation intervention on surgical complications up to a 90-day postoperative period in patients of various surgical disciplines. The hypothesis is that a preoperative smoking cessation program improves outcomes in smokers undergoing intermediate to high-risk surgery.
Methods: The present study is a single-centre, randomized trial with two parallel groups of smokers scheduled for surgery comparing surgery alone and surgery with preoperative smoking cessation. We plan to randomize 251 patients. The primary objective is to compare complications between patients with an institutional multifaceted smoking cessation intervention starting 4 weeks before surgery compared to patients in the advice-only group (control group) within a 90-day postoperative period. The primary endpoint is the Comprehensive Complication Index (CCI®) within 90 days of surgery. Secondary outcomes include the length of hospital stay, cost of care, quality of life, smoking abstinence, and reduction in nicotine consumption.
Discussion: The hypothesis is that a preoperative smoking cessation program improves outcomes in smokers undergoing surgery.
Trial registration: BASEC #2021-02004; ClinicalTrials.gov: NCT05192837. Registered on January 14, 2022.
The published trial protocol can be found here.
Project lead: Ulrike Held & Milo Puhan
Funding: Swiss Cancer League
Reporting and evaluation of prediction models in organ transplantation: a scoping review
Previous research criticized the quality of prediction models concerning poor reporting and the risk of bias. It needs to be investigated how this applies to prediction models in organ donation and transplantation. A scoping review was initiated to assess prediction models currently used in transplant centers in Switzerland, and to update clinicians on the transparency, the quality of reporting, and risk of bias of these tools.
The review protocol is available on OSF: here
Project lead: Simon Schwab & Ulrike Held
Transition to home: improving outcomes of children and families - systematic review
Medical progress has improved markedly the survival rates of very preterm-born infants in recent decades. However, the infants are still at higher risk for long-term impairments as compared to term-born infants. We are performing a systematic review of interventions aiming at improving the transition to home process for very preterm-born infants and their parents.
The protocol was registered on PROSPERO: here
Preprint of the protocol paper is available on medRxiv: here
Project lead: Ulrike Held